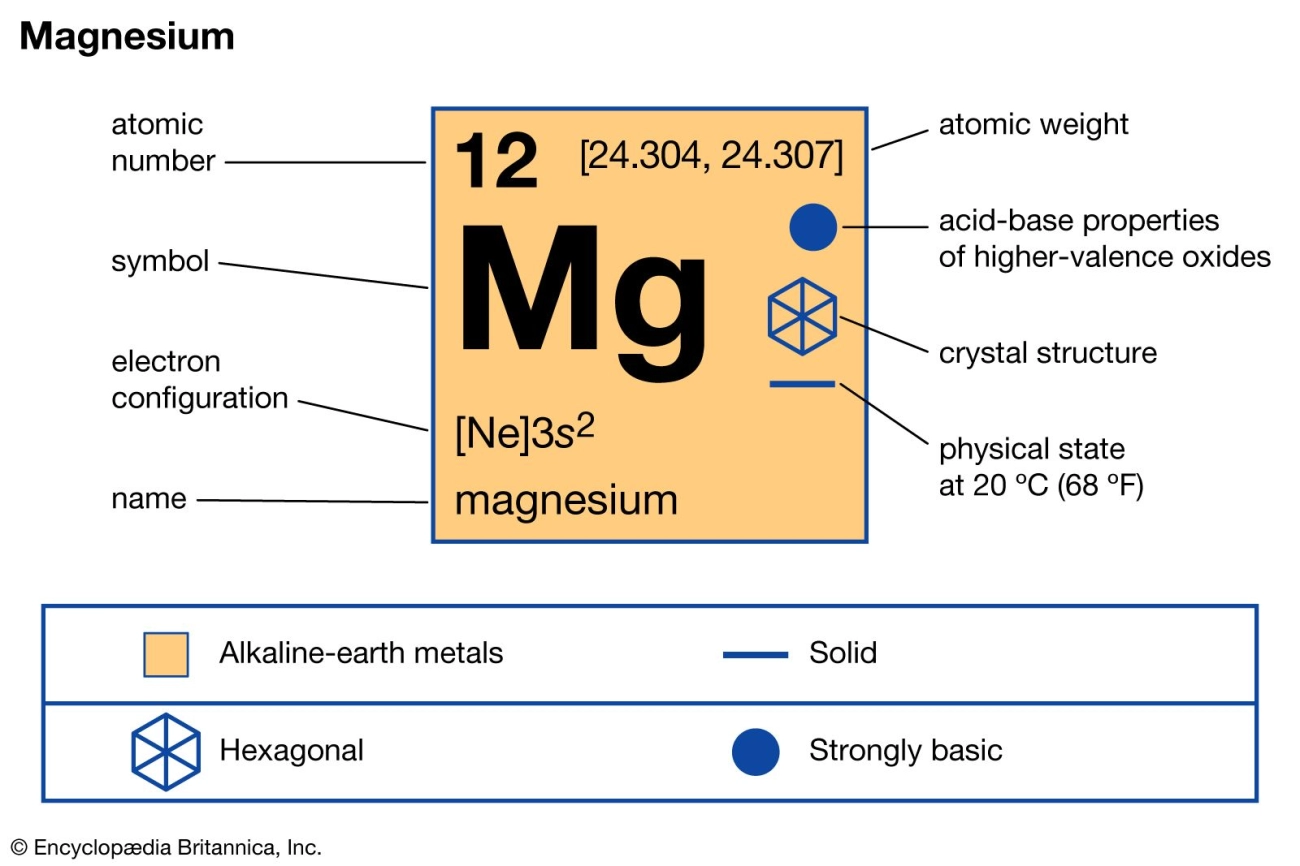
Discover the melting point of magnesium at 650°C and its impact on alloys manufacturing and lightweight metal applications.
What Exactly is Magnesium’s Melting Point and Why Does It Matter
Magnesium’s melting point is the temperature at which this metal changes from a solid to a liquid. Simply put, it’s the point where magnesium absorbs enough heat to break its atomic bonds and become molten. Understanding this phase transition helps engineers and manufacturers know how to handle, shape, and use magnesium effectively. If you look at a phase diagram, the melting point marks the boundary between solid and liquid states under standard pressure.
According to the National Institute of Standards and Technology (NIST), magnesium’s melting point is about 650°C (1202°F). This is a precise value, but small changes can occur depending on the metal’s purity. Impurities and alloying elements can shift this temperature slightly, either raising or lowering the melting threshold.
Historically, magnesium was isolated by Sir Humphry Davy in 1808, who recognized its relatively low melting point compared to many metals. This property allowed early electrolysis methods to work effectively since magnesium melts at a lower temperature than metals like iron or copper.
This relatively low melting point makes magnesium unique—it melts at a much lower temperature than some other structural metals. That opens the door to special heat-sensitive applications and manufacturing processes, which we’ll explore next.
The Science Behind Magnesium’s 650°C Threshold

Magnesium’s melting point sits around 650°C, and this ties closely to its place in the periodic table. As a Group 2 alkaline earth metal, magnesium has two electrons in its outer shell. This electron setup creates relatively weak metallic bonds compared to heavier metals, which helps explain its lower melting temperature. Plus, its low density means atoms aren’t packed super tight, so it takes less heat to break those bonds and turn solid magnesium into liquid.
When we look deeper, magnesium’s thermal properties play a big role too. Its heat of fusion—the energy needed to melt it—is lower than metals like aluminum or titanium, meaning less energy is required to change its phase. Magnesium also boasts good thermal conductivity, which helps it spread heat evenly, preventing hotspots during melting. However, a thin layer of oxide naturally forms on the surface, acting as a protective barrier but sometimes affecting how heat moves during melting or casting.
Other factors can shift magnesium’s melting point a bit. Pressure changes have a minor effect, but impurities and alloying elements are more impactful. For instance, adding aluminum to create a common alloy like AZ91 raises the melting range, improving strength but changing how it melts. Even small amounts of iron or silicon can shift the melting point. Here’s a quick example: pure magnesium melts at 650°C, but AZ91 alloy melts closer to 595–625°C depending on the exact mix.
To put this in perspective, here’s a simple comparison chart of melting points for alkaline earth metals:
| Metal | Melting Point (°C) |
|---|---|
| Beryllium | 1287 |
| Magnesium | 650 |
| Calcium | 842 |
| Strontium | 777 |
| Barium | 727 |
| Radium | 700 (approximate) |
This comparison highlights why magnesium’s melting point is relatively low among its group, making it ideal for applications needing easy melting and shaping without huge energy costs.
Real-World Implications How Magnesium’s Melting Point Shapes Industries
Magnesium’s melting point of about 650°C makes it a favorite in several industries, especially where weight, energy use, and precision matter.
Manufacturing Marvels
Because magnesium melts at a relatively low temperature, die-casting it uses less energy compared to metals like aluminum or steel. This means manufacturers save on costs and reduce their carbon footprint. Custom parts, especially for electronics and machinery, benefit from magnesium’s quick solidification and fine detail capture.
Aerospace and Automotive Edge
Lightweight magnesium alloys, such as AZ91, are popular in vehicles and aircraft. These alloys help make cars and planes lighter, which improves fuel efficiency and lowers emissions—critical for the U.S. market focused on greener transportation solutions.
Challenges and Solutions
Though useful, magnesium’s low melting point also means it’s highly flammable, posing safety risks during melting and casting. To keep things safe, many factories now add rare-earth stabilizers to slow down burning and control ignition, making magnesium processing safer.
Emerging Trends
Magnesium’s characteristics support green technology innovation. It’s being used in recyclable battery casings and in controlled melting processes for cleaner metal extraction. This aligns well with America’s push towards sustainable materials in high-tech industries.
Magnesium vs the Competition Melting Point Showdown
When it comes to melting points, magnesium sits quite low compared to other common metals used in industry. Here’s a quick side-by-side look at magnesium (Mg), aluminum (Al), titanium (Ti), and steel to get a clear picture:
| Metal | Melting Point (°C) | Cost | Energy to Melt (Relative) | Notes |
|---|---|---|---|---|
| Magnesium | 650 | Low | Low | Easy to process but reactive |
| Aluminum | 660 | Moderate | Moderate | Lightweight, corrosion resistant |
| Titanium | 1,668 | High | High | Strong, heat resistant |
| Steel | 1,370 | Moderate | High | Versatile, durable |
Pros and Cons of Magnesium’s Melting Point
Pros:
- Low melting point means less energy used during melting and casting.
- Easier processing for custom and complex shapes.
- Lightweight metal alloys ideal for reducing weight in parts.
Cons:
- The lower melting point makes magnesium more reactive and prone to flammability risks.
- Requires careful handling and safety measures during melting.
- Less heat resistance compared to titanium and steel.
When to Choose Magnesium
Use this quick decision guide to decide if magnesium fits your project needs:
- Weight is a priority: Magnesium’s low density beats steel and titanium.
- Energy efficiency in manufacturing: Use Mg if you want to save power during melting or casting.
- Complex shapes or custom parts: Magnesium die casting temperature suits detailed designs.
- Need high heat resistance or heavy durability? Consider alternatives like steel or titanium instead.
In industries across the U.S., including automotive and aerospace, these factors help manufacturers balance cost, efficiency, and performance. Magnesium’s melting point is a big reason why it continues to be a preferred choice for lightweight, energy-conscious applications.
Safety Handling and Best Practices for Working with Magnesium

When working with magnesium, safety is a must. Magnesium can ignite at temperatures below its melting point (650°C), making it a fire hazard during melting or machining. Here’s a quick risk rundown:
- Ignition risk: Magnesium dust and thin ribbons catch fire easily.
- Handling sparks or flames: Keep away from open flames and avoid grinding that produces dust.
- Use inert gas atmospheres: During melting, protect molten magnesium with argon or nitrogen to reduce oxidation and fire risk.
Pro Tips from Vastpcc for Lab-Scale Melting
If you’re melting magnesium on a small scale, follow these simple steps for safer operations:
- Use a closed furnace with inert gas flow to control the environment.
- Choose crucibles made from graphite or ceramic to withstand heat and prevent contamination.
- Maintain a clean workspace free from dust and flammable materials.
- Have a Class D fire extinguisher ready — special for metal fires like magnesium.
Regulatory Notes for Industrial Users
Compliance is key for U.S. manufacturers and processors handling magnesium:
- Follow OSHA standards for combustible metals, including guidelines on ventilation, storage, and protective equipment.
- Adhere to HazCom rules by properly labeling magnesium materials and providing safety data sheets.
- Train staff regularly on fire prevention and emergency response relevant to magnesium’s unique risks.
By understanding magnesium’s flammability and following these practices, you can safely take full advantage of its low melting point and other useful properties in industrial or lab environments.
Frequently Asked Questions Magnesium Melting Point Edition
Here are some common questions about magnesium’s melting point to help you get clear answers fast.
Does alloying magnesium change its melting point?
Yes. Adding elements like aluminum or zinc can raise or lower the melting point slightly depending on the alloy. For example, AZ91 (a popular magnesium alloy) melts around 595°C, a bit lower than pure magnesium’s 650°C.
How does magnesium’s melting point compare to other metals?
Magnesium melts at about 650°C, which is low compared to aluminum (660°C) and much lower than steel (around 1370°C) or titanium (1668°C). This makes magnesium easier to melt and cast with less energy.
What is magnesium’s boiling point?
Magnesium boils at roughly 1090°C. This is important for high-temp industrial processes where you want to avoid vaporizing the metal.
Can I search for magnesium melting point by voice?
Yes. Simple voice commands like “what is magnesium melting point” or “melting point magnesium factory in China” will usually get you quick, accurate results.
Does purity affect magnesium’s melting point?
Absolutely. Higher purity magnesium melts closer to 650°C, while impurities or recycled materials may cause minor melting point shifts.
If you need more info or want help sourcing magnesium with specific properties for your project, especially from trusted US manufacturers or China-based factories, feel free to ask!

