Discover what metalloids are their unique properties examples and key roles in science and technology explained for students and enthusiasts.
Metalloids Explained Properties Examples and Uses in Science
Discover what metalloids are their unique properties examples and key roles in science and technology explained for students and enthusiasts.
What Are Metalloids
Metalloids are elements that have properties intermediate between metals and nonmetals. In chemistry, they are often called semimetals because they don’t fit cleanly into either category. Scientifically, metalloids exhibit mixed characteristics, which means they can behave like metals in some situations and like nonmetals in others.
On the Periodic Table, metalloids are positioned along a distinct stair-step line that separates metals on the left from nonmetals on the right. This diagonal boundary helps identify elements with semimetallic traits. Because of their unique location, metalloids often show moderate electrical conductivity, earning them the name semiconductors.
Understanding where metalloids fall on the Periodic Table helps explain their hybrid nature, blending metallic luster with nonmetal-like chemical behavior. This blend makes them crucial in various scientific and industrial applications.
Physical and Chemical Properties of Metalloids

Metalloids have physical and chemical properties that sit between metals and nonmetals, which is why they are often called semimetals. Their electrical conductivity is one of the biggest clues—unlike metals, metalloids don’t conduct electricity as well, but unlike nonmetals, they still conduct some electricity, making them useful as semiconductors.
In terms of appearance, metalloids usually look like shiny metals but can be brittle instead of malleable. They are harder than most nonmetals but not as tough as most metals. Their melting and boiling points vary widely but generally fall between those of metals and nonmetals, reflecting their intermediate nature.
Chemically, metalloids can react in ways similar to both metals and nonmetals. For example, they might form alloys like metals or bond with nonmetals in compounds. This dual behavior is why they are so important in various chemical and industrial processes.
List of Common Metalloids and Their Characteristics
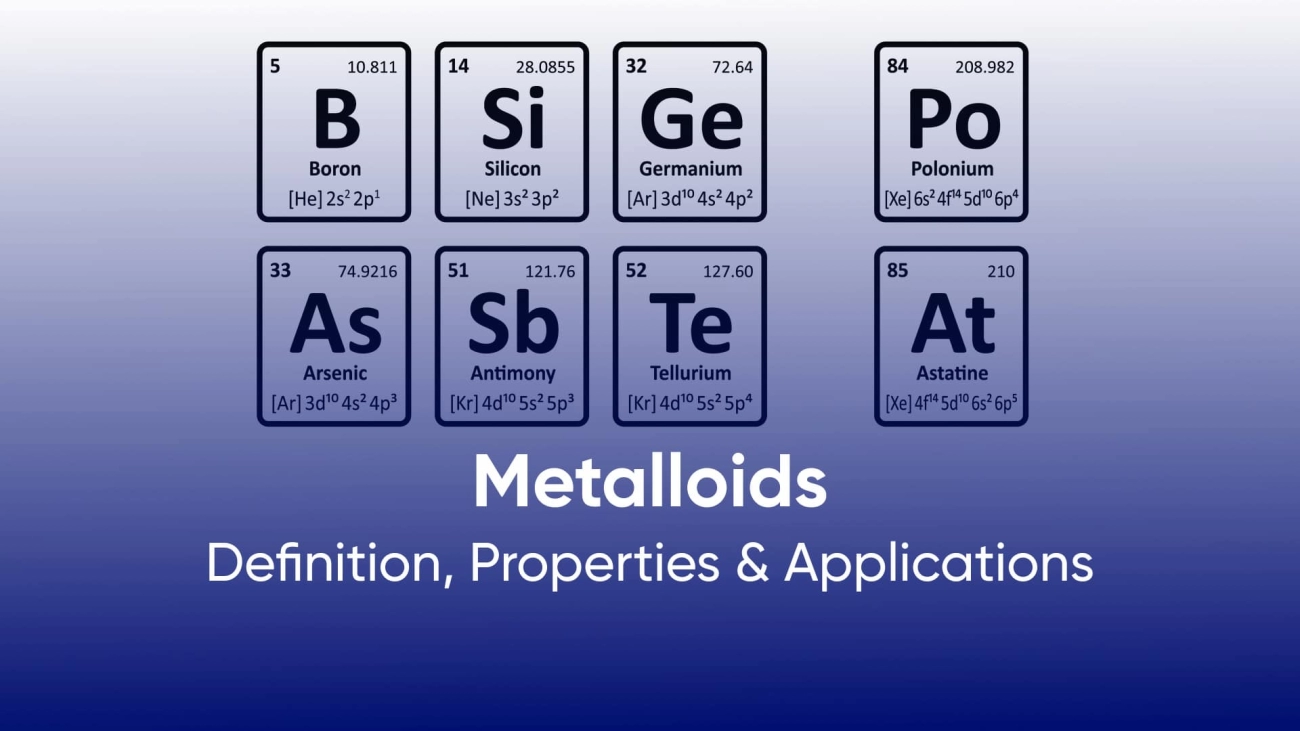
Here are some of the well-known metalloids and what makes each unique:
- BoronKnown for its hardness and heat resistance, boron is vital in making strong, lightweight materials like borosilicate glass and fiberglass. It’s also used in detergents and as a neutron absorber in nuclear reactors.
- SiliconThe most famous metalloid, silicon is essential for electronics and semiconductors. It’s the backbone of computer chips and solar panels, making it crucial for modern technology.
- ArsenicWhile toxic in large amounts, arsenic finds uses in pesticides, wood preservatives, and certain alloys. It also has historical significance in medicine and glass coloring.
- AntimonyAntimony is mainly used to harden metals like lead in batteries and ammunition. It also serves as a flame retardant in textiles and plastics.
- TelluriumRare and brittle, tellurium is used in solar cells, thermoelectric devices, and as an alloy additive to improve metal properties. It also helps improve the efficiency of semiconductors.
- PoloniumA radioactive metalloid, polonium has limited uses mostly in scientific research and as a heat source in space equipment. Its rarity and radioactivity make it less common in everyday applications.
- Germanium (sometimes classified as a metalloid)Germanium shares many semiconductor properties with silicon. It’s used in fiber optics, infrared optics, and transistors, supporting advancements in telecommunications.
Each of these metalloids blends metallic and nonmetallic traits in ways that make them valuable across different industries, especially in American tech and manufacturing markets.
Metalloids vs Metals and Nonmetals Key Differences
Metalloids sit between metals and nonmetals on the periodic table, showing traits from both groups. Here’s a quick breakdown of how they compare:
| Property | Metals | Metalloids | Nonmetals |
|---|---|---|---|
| Electrical Conductivity | High (good conductors) | Moderate (semiconductors) | Low (poor conductors) |
| Appearance | Shiny and lustrous | Dull to shiny | Dull |
| Malleability | Malleable and ductile | Brittle | Brittle |
| Chemical Behavior | Tend to lose electrons (form cations) | Can lose or gain electrons | Tend to gain electrons |
| Position on Periodic Table | Left and center | Along the stair-step line | Right side |
| Common Uses | Structural materials, wiring | Semiconductors, alloys | Insulators, gases |
Role in Chemical Bonding
- Metals usually form ionic bonds by giving up electrons.
- Nonmetals tend to gain or share electrons, forming covalent bonds.
- Metalloids can do both, depending on the element they react with, making them versatile in creating different types of bonds.
This unique mix of properties is why metalloids, or semimetals, are essential in industries like electronics, where controlled conductivity matters. They bridge the gap between metals’ conductivity and nonmetals’ insulating abilities, making them perfect for semiconductors and advanced materials.
Applications and Uses of Metalloids
Metalloids play a big role in many industries, thanks to their unique mix of metal and nonmetal properties. Here’s a quick look at how some common metalloids are used:
- Silicon
- The backbone of electronics and semiconductor technology
- Used in making computer chips, solar panels, and almost all modern gadgets
- Its semiconducting nature allows it to control electrical currents efficiently
- Arsenic
- Added to pesticides to protect crops
- Used in alloys to strengthen metal products like lead shot and bullets
- Sometimes used in wood preservatives and semiconductor devices
- Boron
- Key ingredient in borosilicate glass, which is heat-resistant and used in cookware and lab equipment
- Found in detergents and bleaches to boost cleaning power
- Used in fiberglass and as a neutron absorber in nuclear reactors
Emerging tech is also exploring metalloids for new materials, like advanced semiconductors and energy storage devices. Their unique properties make them essential for innovation in electronics, green energy, and materials science.
Metalloids in Everyday Life

Metalloids play a big role in the technology and products we use daily. Because they have both metal and nonmetal traits, they offer unique benefits in many areas.
How Metalloids Impact Modern Technology
- Silicon is the star here. It’s the base for most computer chips and smartphones because it acts as a reliable semiconductor.
- Boron is often used to make strong, heat-resistant glass like the kind in ovenware or smartphone screens.
- Arsenic pops up in tiny amounts in some agricultural pesticides and specialized alloys.
- Antimony helps increase hardness in batteries and flame retardants.
Examples of Metalloids in the Home and Industry
- Silicon in your phone, laptop, and solar panels.
- Boron in laundry detergents and fiberglass insulation.
- Tellurium in thermoelectric devices that convert heat to electricity.
- Germanium (sometimes classed as a metalloid) is used in fiber optic systems and infrared optics.
These metalloids are behind many gadgets, building materials, and everyday products, quietly making our lives easier and more connected.
Fun Facts and Myths about Metalloids
Metalloids often get misunderstood because they don’t fit neatly into the metal or nonmetal categories. One common myth is that metalloids are just “half metals,” but they actually have unique properties that set them apart as semimetals. For example, people sometimes think all metalloids are good conductors like metals, but their electrical conductivity can vary widely—some act like semiconductors, which is why they’re key in electronics.
Historically, metalloids like arsenic were famous (or infamous) for their toxic uses, which gave these elements a bit of a shady reputation. Yet, elements like silicon quietly revolutionized technology behind the scenes by making computers, smartphones, and solar panels possible.
Another fun fact: the stair-step line on the periodic table isn’t just a divider; it highlights how metalloids bridge two very different worlds in chemistry, making them essential yet unique. People sometimes confuse germanium as a metalloid, but its classification can vary depending on the source.
Understanding these myths helps clear up the real value of metalloids—they’re not just “in-between” elements, but vital parts of our modern lives and technologies.
